Recently, the State Key Laboratory of heavy oil reported new research results called “Dense CO Adlayers as Enablers of CO Hydrogenation Turnovers on Ru Surfaces”, which published on the Journal of the American Chemical Society (JACS). Chemical engineering and technology student Liu Jianwei is the first author of the paper and China University of Petroleum (East China) is the first signature unit.
At present, the global oil resources gradually developed heavy quality, poor quality direction and the supply is experiencing sustained tension. Foreign large energy companies are vigorously developing coal, natural gas and other conventional energy as raw material Fischer-Tropsch synthesis technology for energy security.
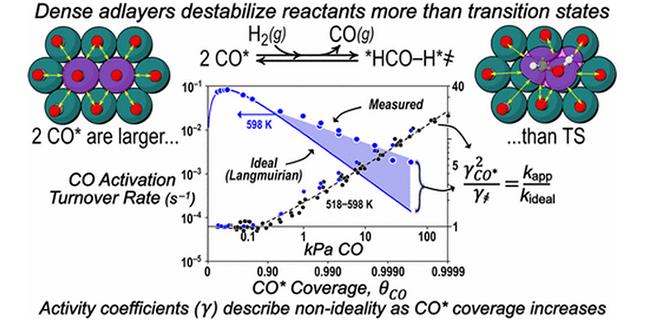
In recent years, the mechanism of Fischer-Tropsch synthesis reaction used surface chemistry research methods has been explored the metal single crystal catalyst surface CO adsorption and CO hydrogenation reaction behavior. However, the results of these studies are quite different from the Fischer-Tropsch synthesis reaction under the actual reaction conditions. Numerous studies have shown that CO has a strong ability to adsorb on metal surfaces. Under the actual Fischer-Tropsch synthesis reaction conditions, the surface of the metal catalyst is substantially covered by the adsorbed CO to form a dense CO adsorption layer. This adsorption behavior may lead to the difference between the above surface chemistry results and the actual reaction process.
Liu Jianwei et al. studied the effect of CO surface coverage on CO adsorption and CO hydrogenation process on the surface of supported ruthenium based catalyst under the actual reaction conditions in the range of wide CO pressure by in situ infrared and kinetic experiments. The results show that there is a large difference in the adsorption behavior of CO on the catalyst surface in different CO pressure ranges. Under the condition of higher CO pressure, the surface coverage of CO on the catalyst surface is close or even higher than that of monolayer adsorption to form a dense CO adsorption layer which changes the relative stability of the adsorbed species on the catalyst surface, thus promoting the CO hydrogenation process. At the same time, the above-mentioned promotion is explained by the transition state theory. In the study, supported metal catalysts and the reaction conditions close to the actual Fischer-Tropsch synthesis process were used. The experimental results can be more truly reflected in the actual reaction process, although the complexity of the research system is increased. The results of this study explain the inhibitory effect of CO pressure on the reaction process of Fischer-Tropsch synthesis under the actual reaction conditions, and provide a theoretical basis for solving the specific problems in the actual industrial process. At the same time, the paper also participates provides a research ideas to reaction process of other strongly adsorbed species.
The results of this research have been affirmed by anonymous reviewers. The reviewers have spoken highly of the experimental ideas and the theoretical methods used in the paper. The research results are of great significance to the deep understanding of many reactions including Fischer-Tropsch synthesis.
American Chemical Society(JACS) founded in 1879 has been one of the most prestigious academic magazines in the field of global chemistry and represents the academic and authoritative academic point of view of the world's chemical industry. Unique academic status and academic value are widely concerned by the chemical field. the latest impact factor in 2016 was 13.858.